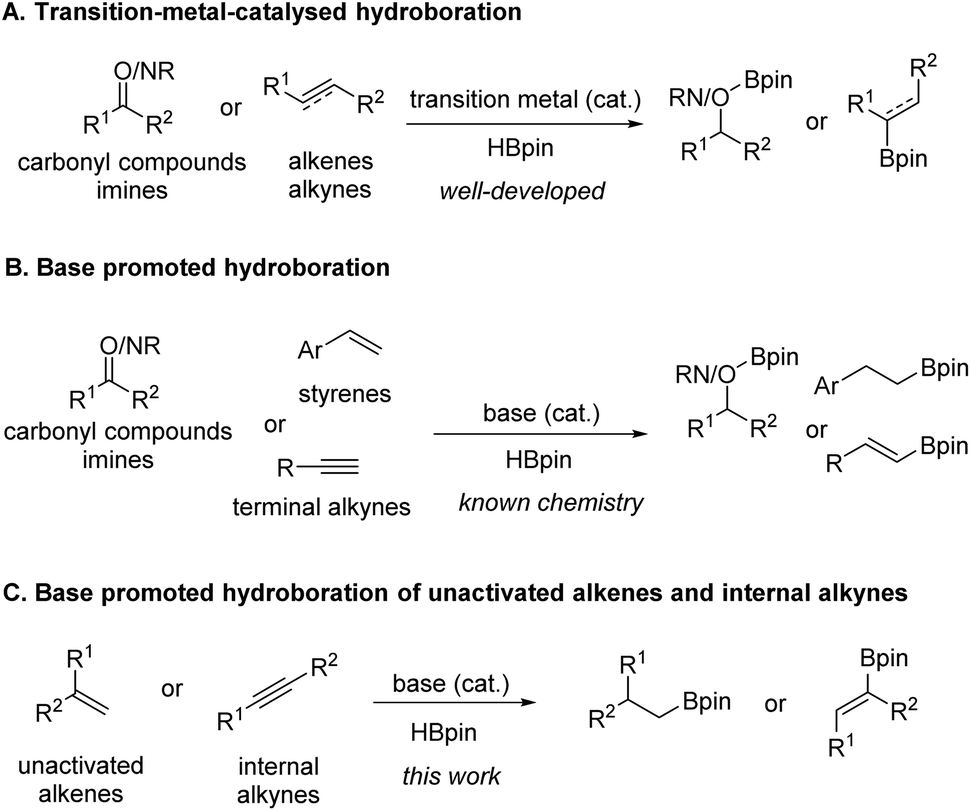
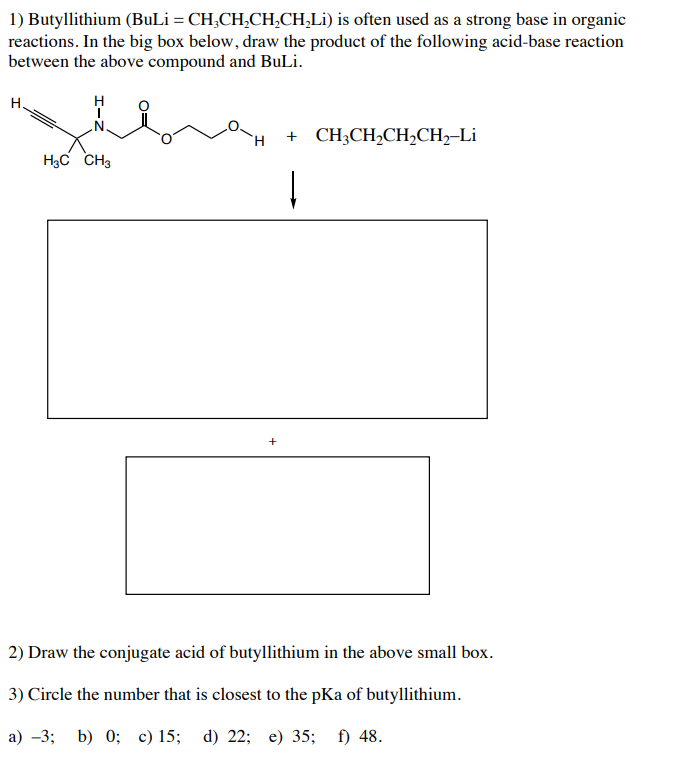
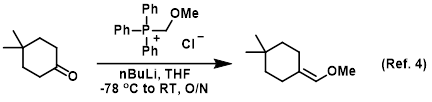n-Butyllithium/N,N,N',N'-Tetramethylethylenediamine-Mediated Ortholithiations of Aryl Oxazolines: Substrate-Dependent Mechanisms | Journal of the American Chemical Society

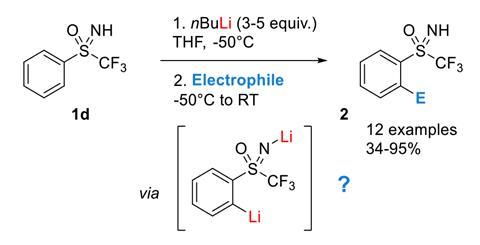
NMR and DFT Studies with a Doubly Labelled 15N/6Li S‐Trifluoromethyl Sulfoximine Reveal Why a Directed ortho‐Lithiation Requires an Excess of n‐ BuLi - Hédouin - Angewandte Chemie International Edition - Wiley Online Library

n-BuLi as a Highly Efficient Precatalyst for Hydrophosphonylation of Aldehydes and Unactivated Ketones | Organic Letters

Scheme 1. Different reactions of N, N-dimethylbenzylamine with n-BuLi /... | Download Scientific Diagram

Mechanism of the Deprotonation Reaction of Alkyl Benzyl Ethers with n‐Butyllithium - Raposo - 2013 - Chemistry – A European Journal - Wiley Online Library

Asymmetric Deprotonation using s-BuLi or i-PrLi and Chiral Diamines in THF: The Diamine Matters | Journal of the American Chemical Society