FE-SEM image of morphology of BALB/3T3 cells on different scaffolds... | Download Scientific Diagram
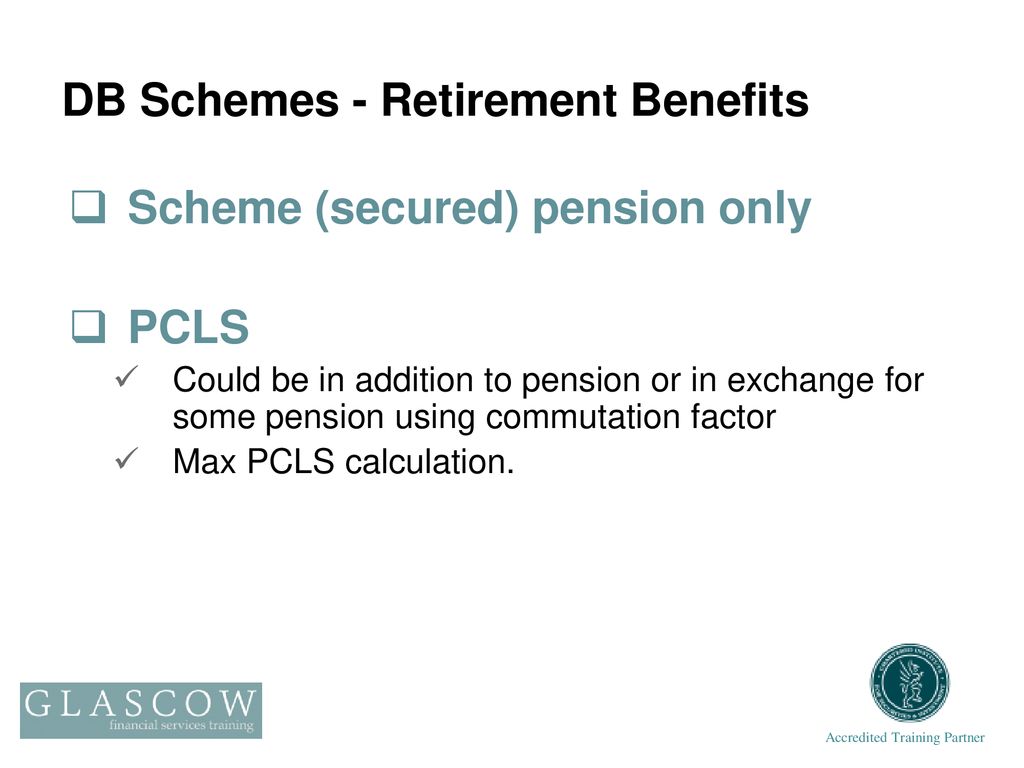
SOLVED: CHEMWORK Calculate AH for the reaction PClz(D) + Clz(g) = PCls(s) given the following data: Equation AH P4(s) + 6 Clz(g) 4 PClz() -1280 kJ Pa(s) + 10 Clz(g) - 4
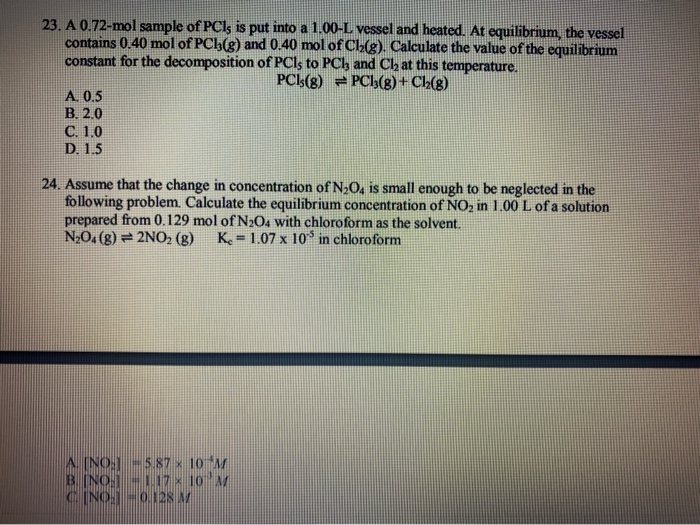
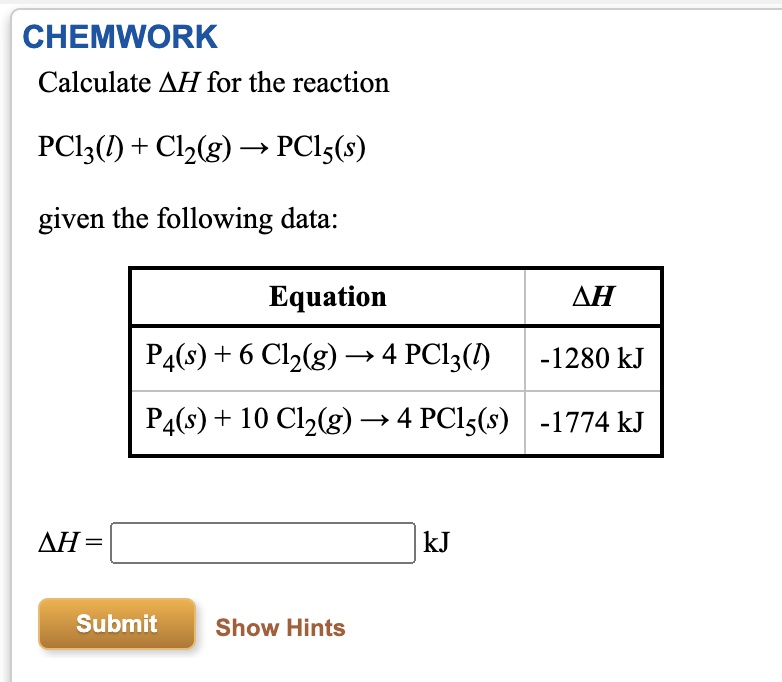
SOLVED: 1. 6.19 grams sample of PCls is placed in an empty 2.00 Liter flask and completely vaporized at 252C. Calculate the pressure in the flask if no chemical reaction were to
![SOLVED: 1o. Calculate PCls for the reaction below assuming that the equilibrium concentrations and equilibrium constant are as follows: Ko = 0.0042, [PCls] = 0.10M, [CI] = 0.10M PC15g=PC13g+Cl2g 11. Calculate [HCl] SOLVED: 1o. Calculate PCls for the reaction below assuming that the equilibrium concentrations and equilibrium constant are as follows: Ko = 0.0042, [PCls] = 0.10M, [CI] = 0.10M PC15g=PC13g+Cl2g 11. Calculate [HCl]](https://cdn.numerade.com/ask_images/04fdb71cae024bca934df0c5d38a25f1.jpg)
SOLVED: 1o. Calculate PCls for the reaction below assuming that the equilibrium concentrations and equilibrium constant are as follows: Ko = 0.0042, [PCls] = 0.10M, [CI] = 0.10M PC15g=PC13g+Cl2g 11. Calculate [HCl]
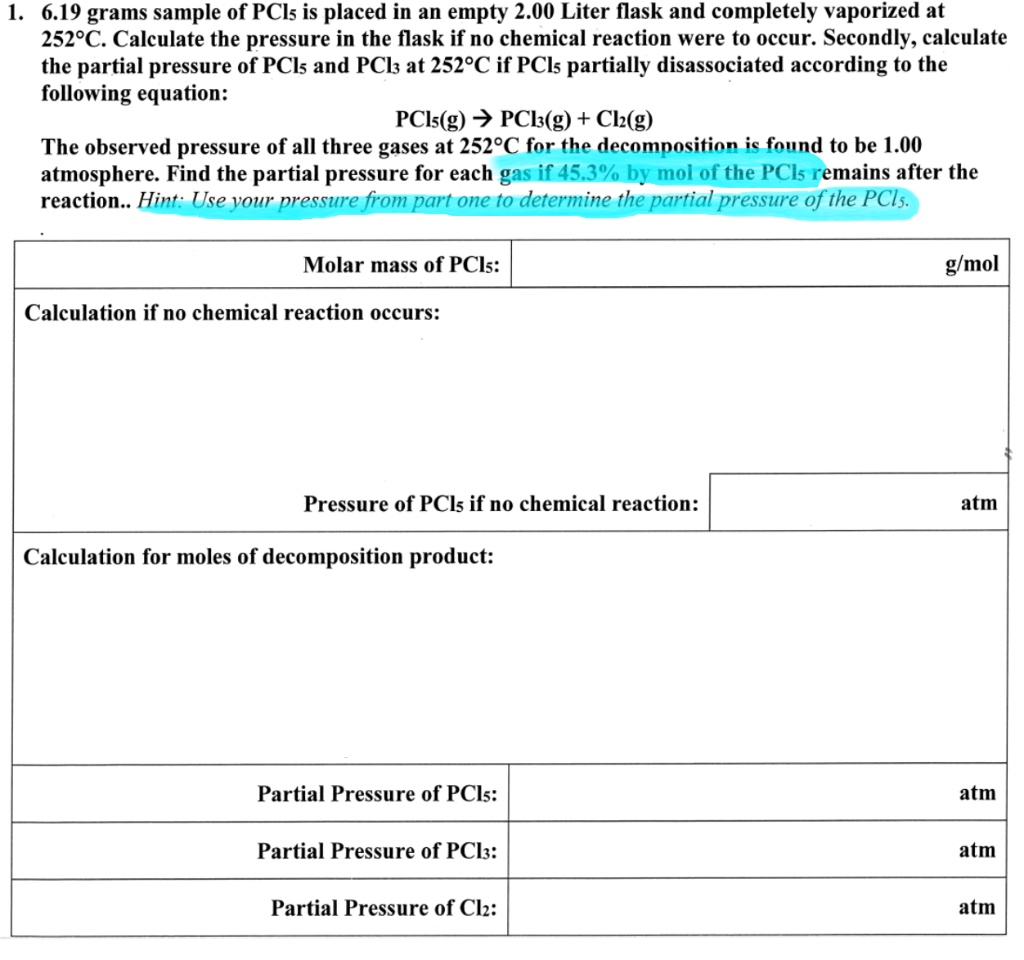
SOLVED: Consider the following reaction: PCl3 (g) Cl2 (g) PCls (9) Calculate the equilibrium constant for this reaction at 800 K by using the following data: Substance GG (kJ/mol) f PCla Clz
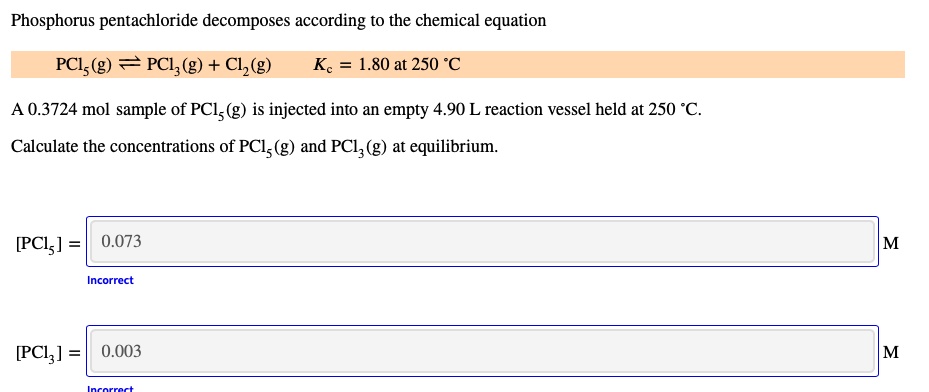
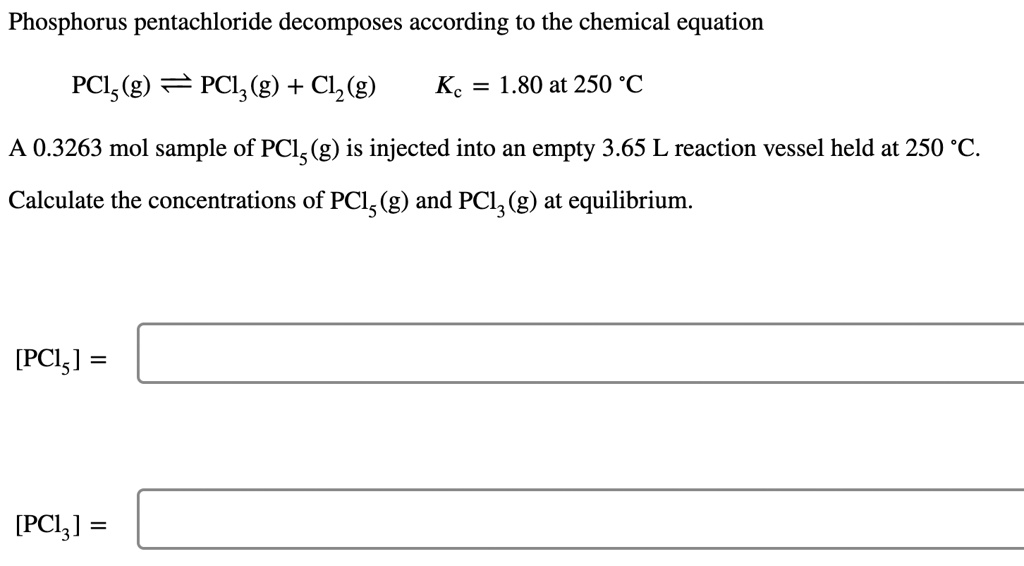
SOLVED: Phosphorus pentachloride decomposes according to the chemical equation PCls (g) = PClz(g) + C1z (g) Kc = 1.80 at 250 *C A 0.3724 mol sample of PCl; (g) is injected into
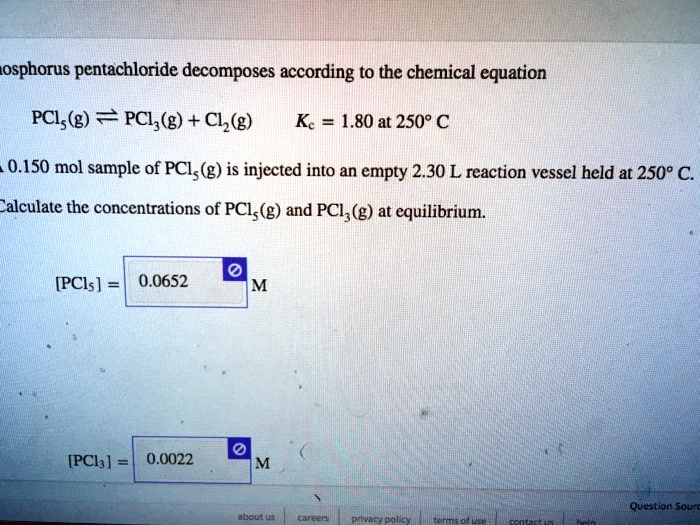
SOLVED: osphorus pentachloride decomposes according to the chemical equation PCls(g) == PCl,(g) + Cl,(g) Kc 1.80 at 2509 € 0.150 mol sample of PCIs(g) is injected into an empty 2.30 L reaction
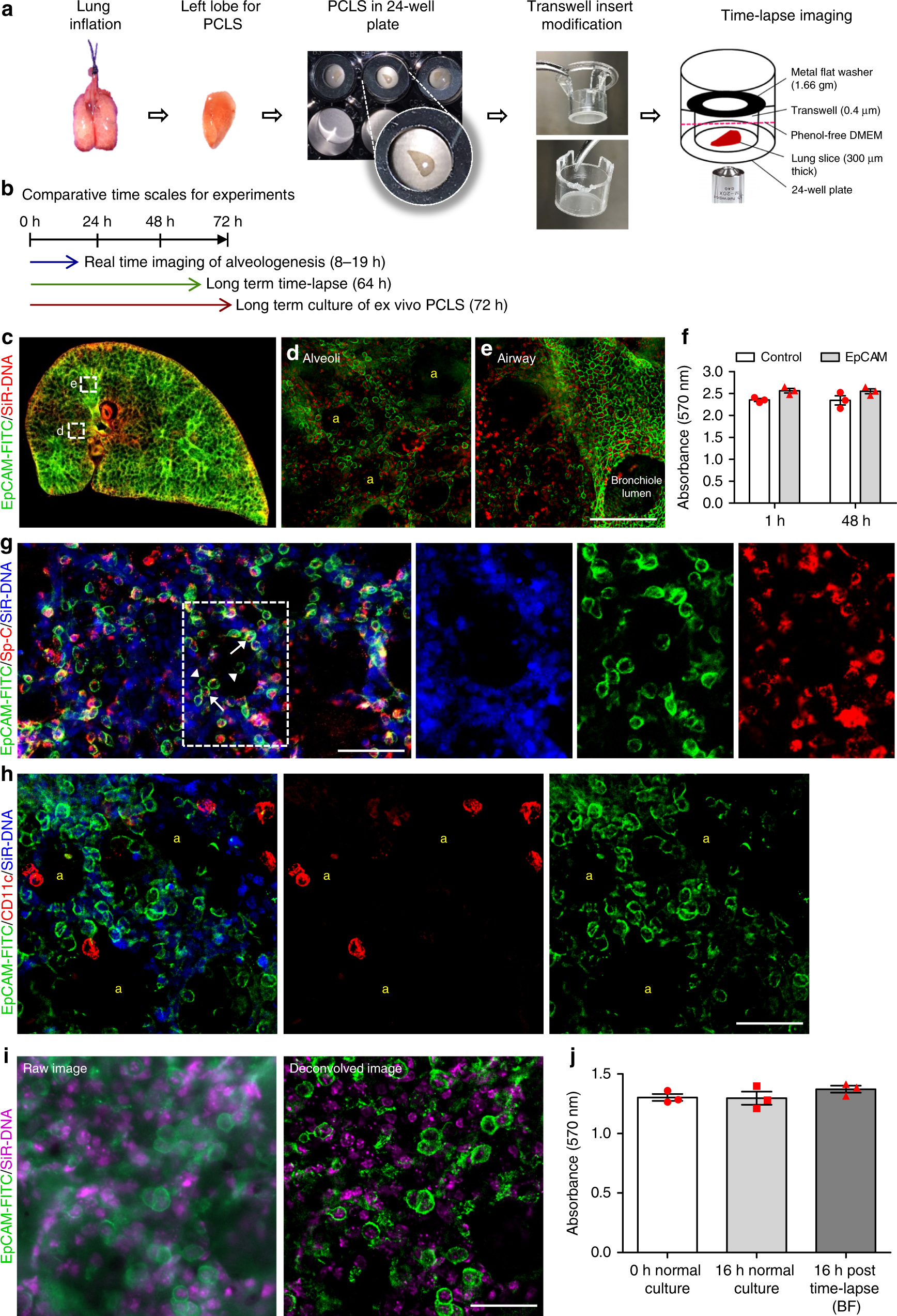
Live imaging of alveologenesis in precision-cut lung slices reveals dynamic epithelial cell behaviour | Nature Communications