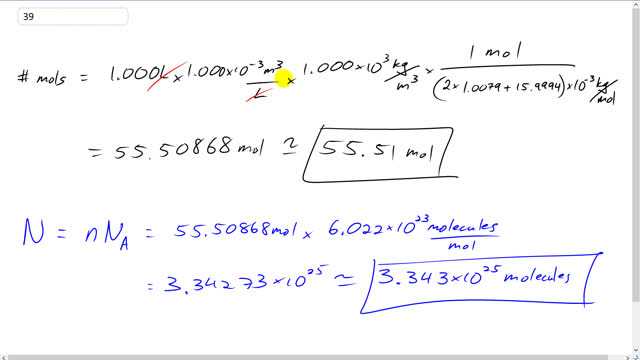
calculate the no of moles present in 1 litre of water if the dw c alculate the no of moles present in 1 l of water if the density of water is

SOLVED: Mole-Volume Conversions Determine the volume in liters occupied by 0.030 moles of a gas at STP. How many moles of argon atoms are present in 11.2 L of argon gas at
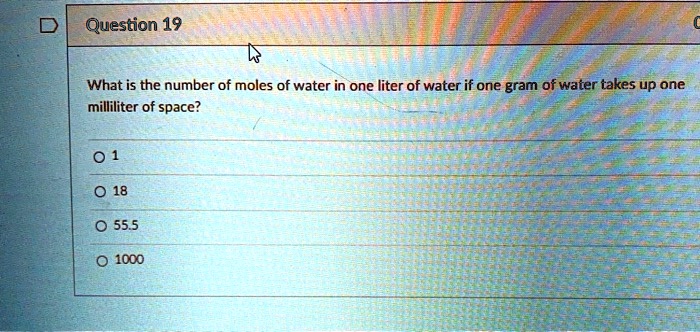
SOLVED: Question 19 What is the number of moles of water in one liter of water if one gram of water takes up one milliliter of space? 0 1 0 18 0 555 I000
The number of water molecules in 1 litre of water is - Sarthaks eConnect | Largest Online Education Community

Calculate the number of moles in 1 L of water (Density of water 1 g/mL). Also calculate the number of water - Brainly.in
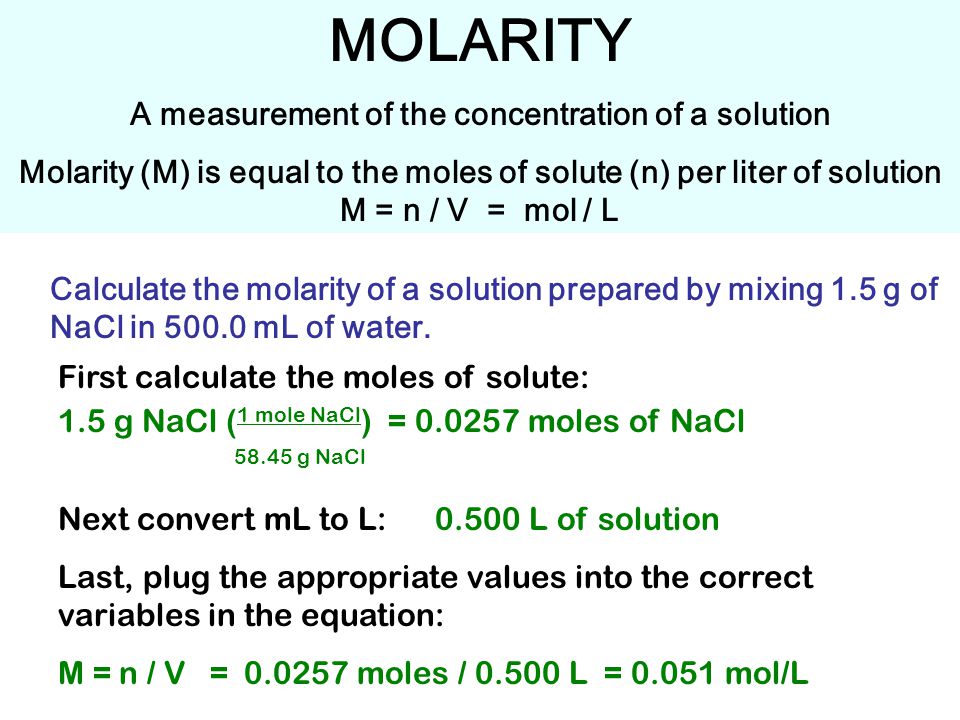
MOLARITY A measurement of the concentration of a solution Molarity (M) is equal to the moles of solute (n) per liter of solution M = n / V = mol / L Calculate. - ppt download
How many liters of water can be made from 55 grams of oxygen gas and an excess of hydrogen at a pressure of 12.4 atm and a temperature of 85C? | Socratic


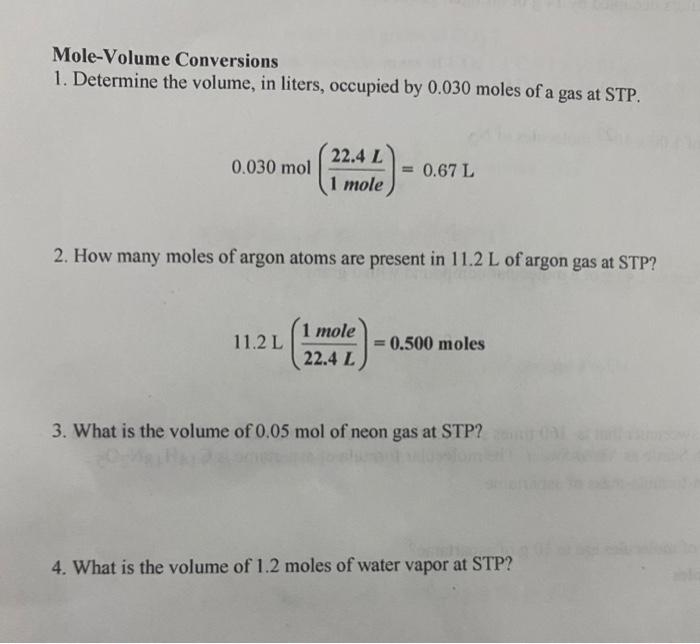






:max_bytes(150000):strip_icc()/GettyImages-692027135-419fe3ddc26e4415b356380582c4e5b2.jpg)
