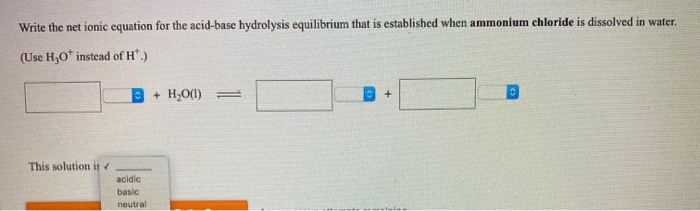
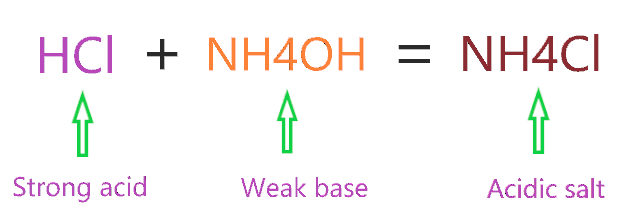
pH calculation of a buffer solution made from a weak base and its conjugate acid (salt form) - YouTube

SOLVED:A buffer contains significant amounts of ammonia and ammonium chloride. Write equations showing how this buffer neutralizes added acid and added base.

Write the chemical formula of ammonium chloride. Explain why an aueous solution of ammonium chloirde - YouTube

Ammonium-chloride-assay - Assay Of Ammonium Chloride AIM: To determine the percentage purity of - Studocu

SOLVED: In a blood buffer, ammonium chloride (NH4Cl) was added to lower the blood pH. The ammonium ion is what acts as the acid. The chloride ion does not have any acid/base
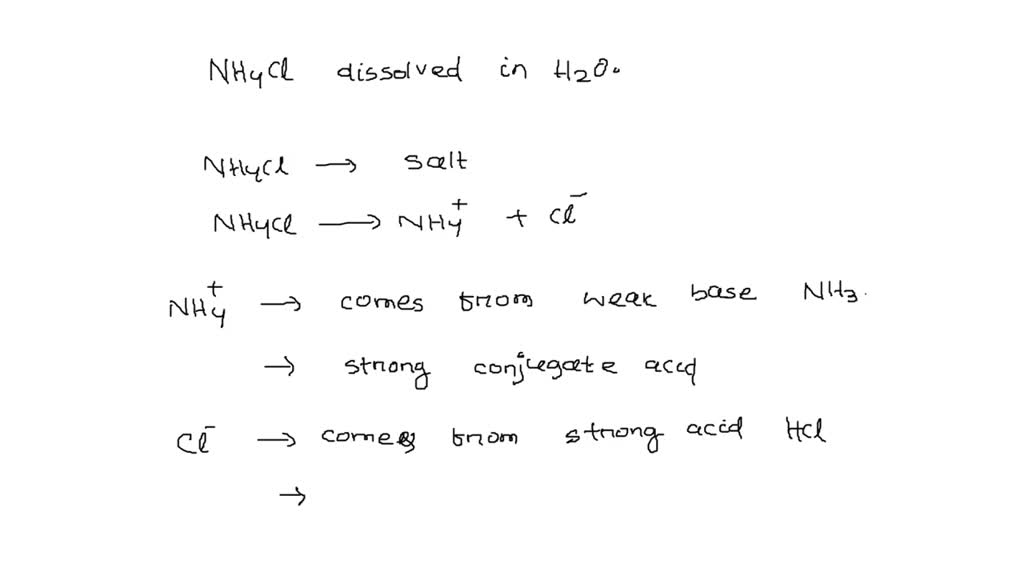
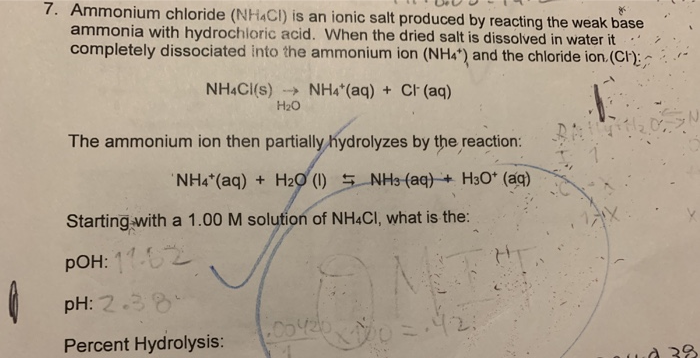
![Solved BUFFERS 10. An ammonium[NH3]/ammonium chloride | Chegg.com Solved BUFFERS 10. An ammonium[NH3]/ammonium chloride | Chegg.com](https://media.cheggcdn.com/media/98f/s907x655/98fc93a3-c6f6-4a89-8acb-45d7d9f5eb4d/image.png)