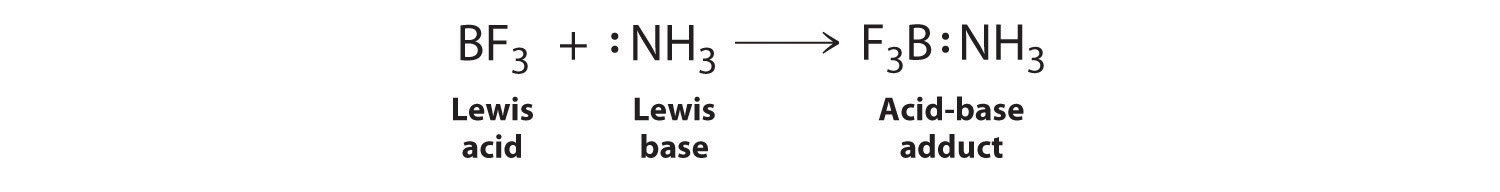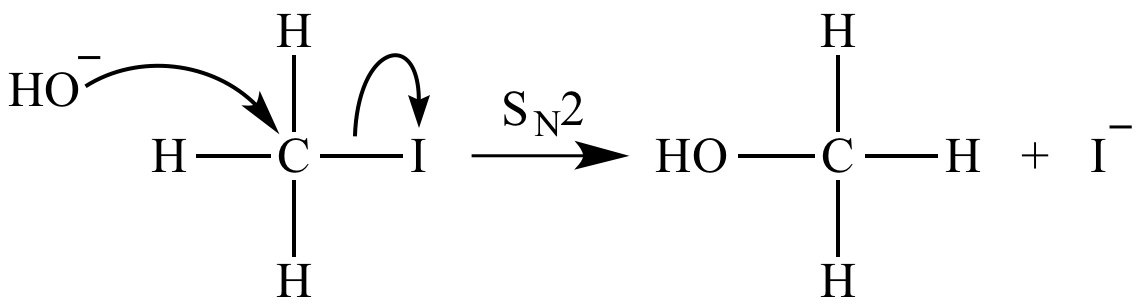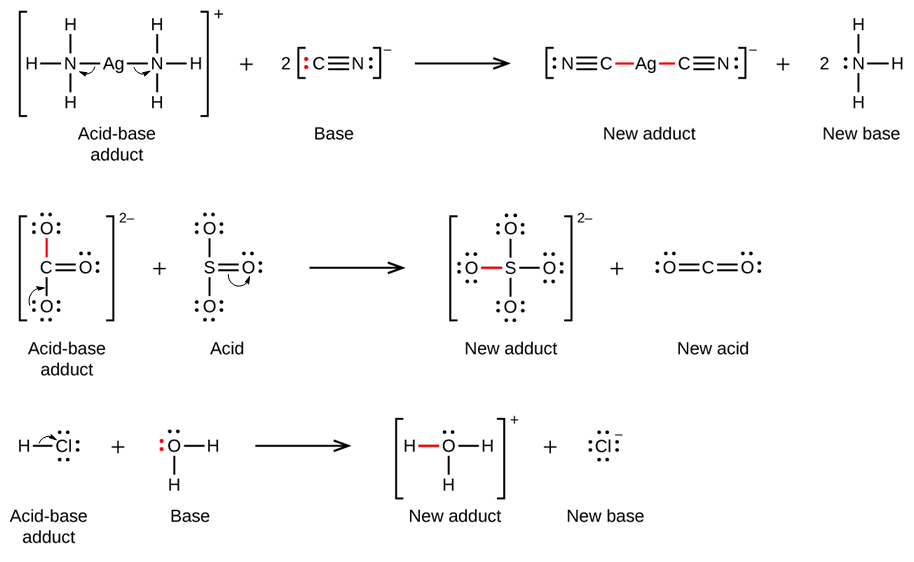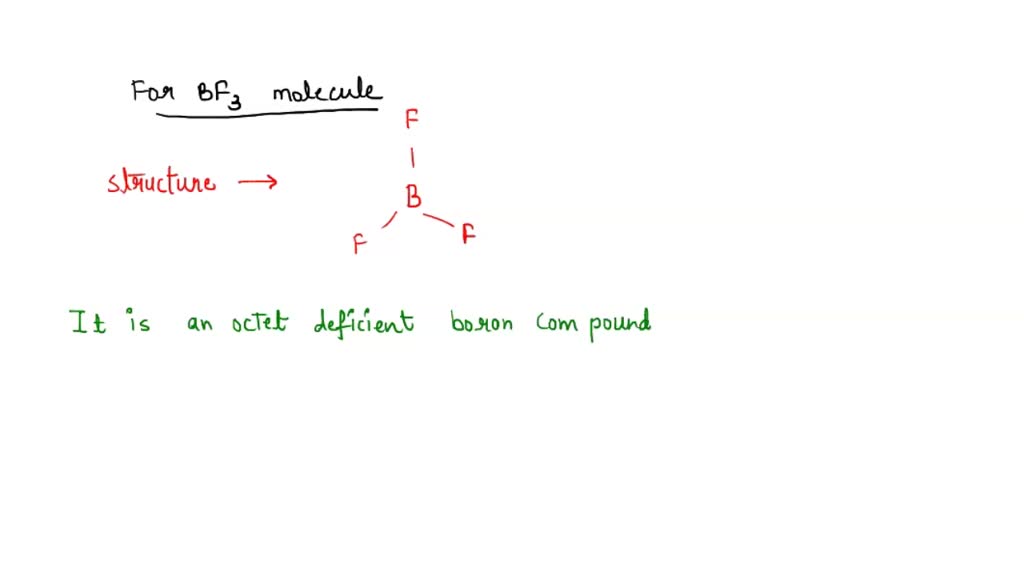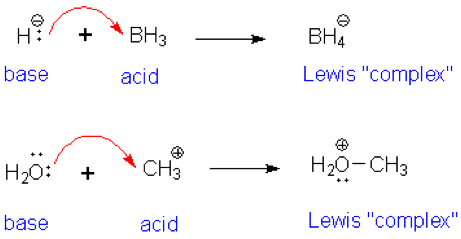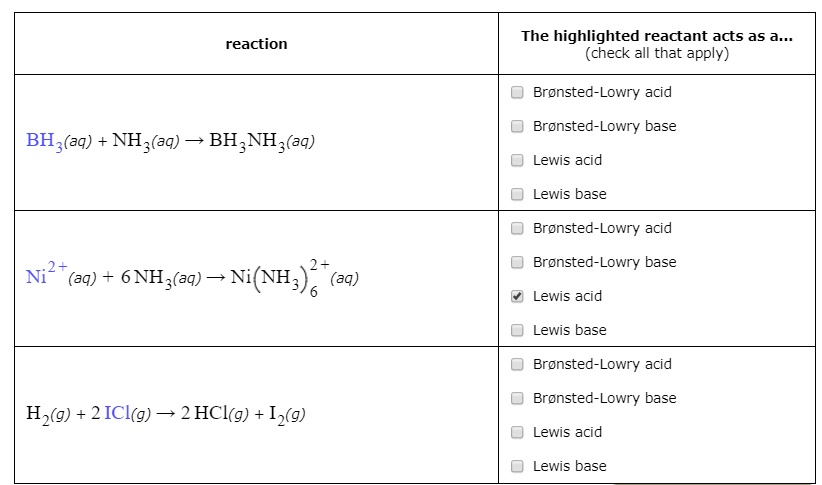
SOLVED: The highlighted reactant acts as a (check all that apply) reaction Bronsted-Lowry acid Bronsted-Lowry base BHz(aq) NHz(aq) BH3NH3(aq) Lewis acid Lewis base Bronsted-Lowry acid Bronsted-Lowry base Ni (aq) 6 NH3(aq) Ni(NH;) (
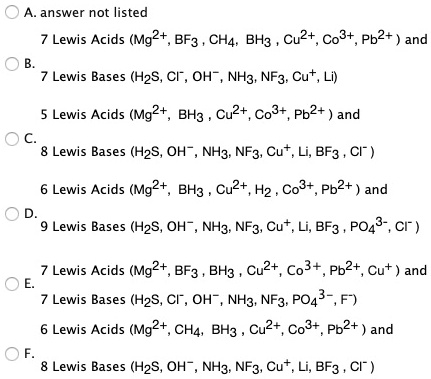
SOLVED: answer not listed Lewis Acids (Mg2+, BF? CHA; BH3 Cu2+, Co3+, Pb2+ and Lewis Bases (H2S, Cl , OH- NH3; NF3; Cut Lewis Acids (Mg2+, BH3 Cuz+,Co3+, Pb2+ and Lewis Bases (

Nature and Strength of Lewis Acid/Base Interaction in Boron and Nitrogen Trihalides - Rodrigues Silva - 2020 - Chemistry – An Asian Journal - Wiley Online Library
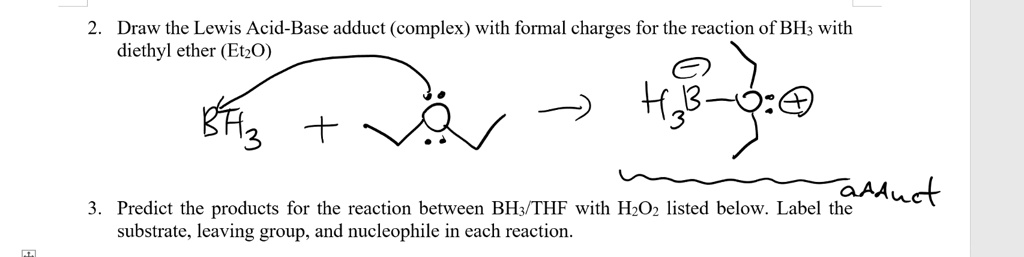
SOLVED: Draw the Lewis Acid-Base adduct (complex) with formal charges for the reaction of BH; with diethyl ether (EtzO) aAAuct Predict the products for the reaction between BH3 THF with HzOz listed