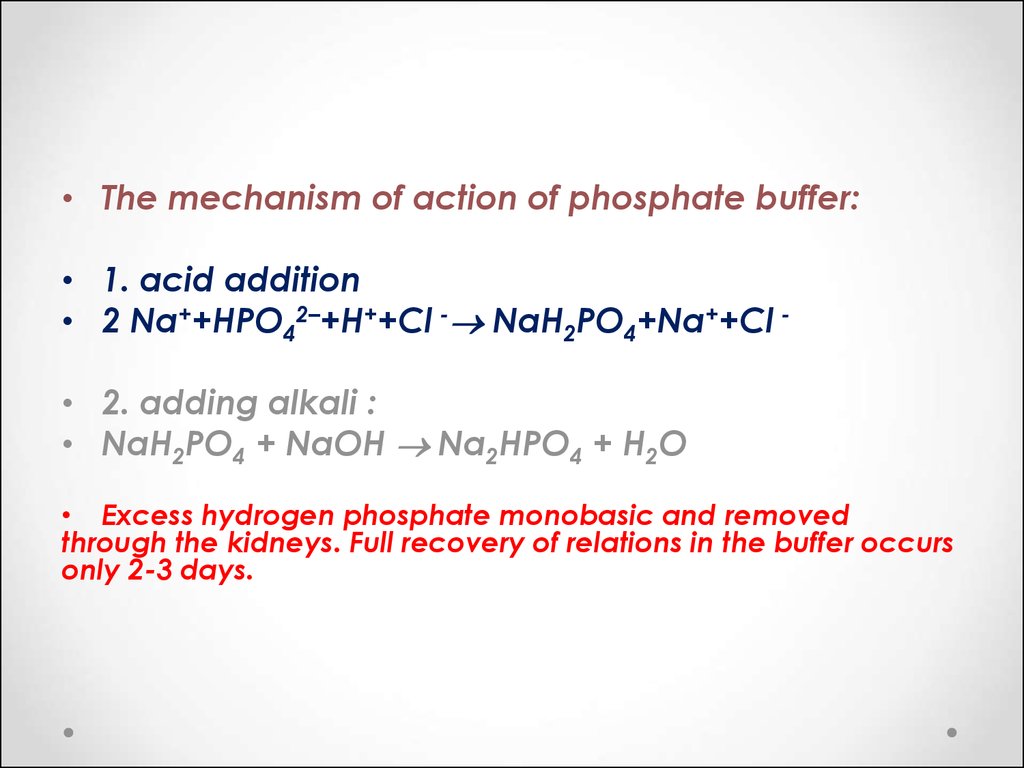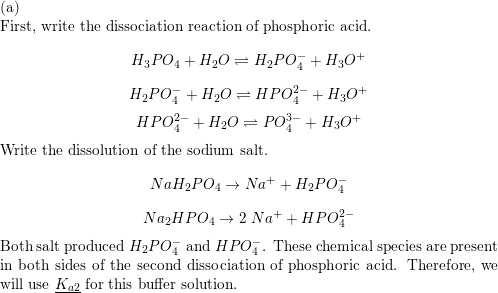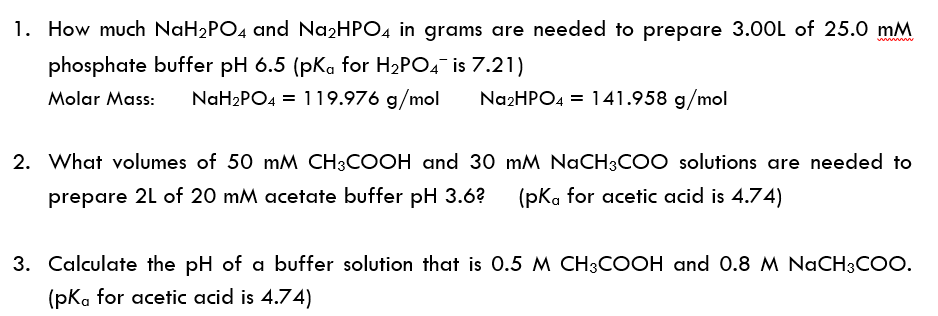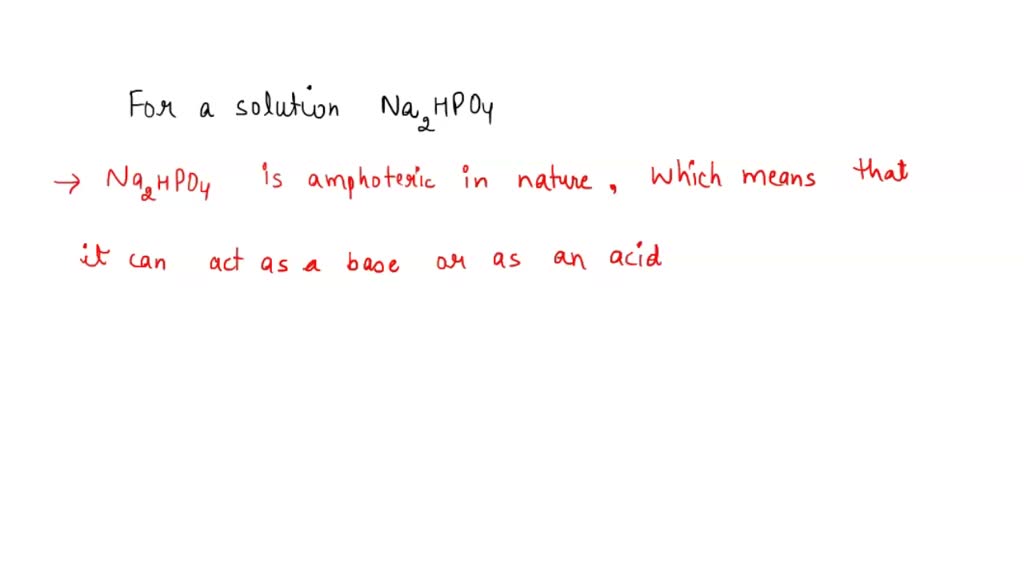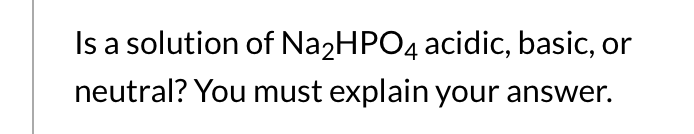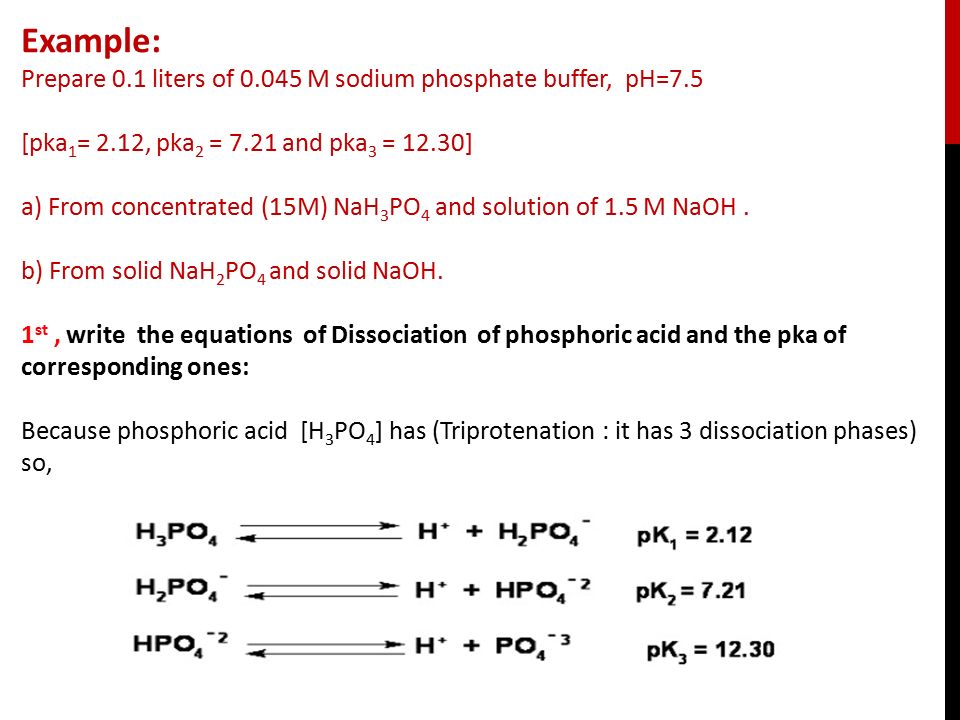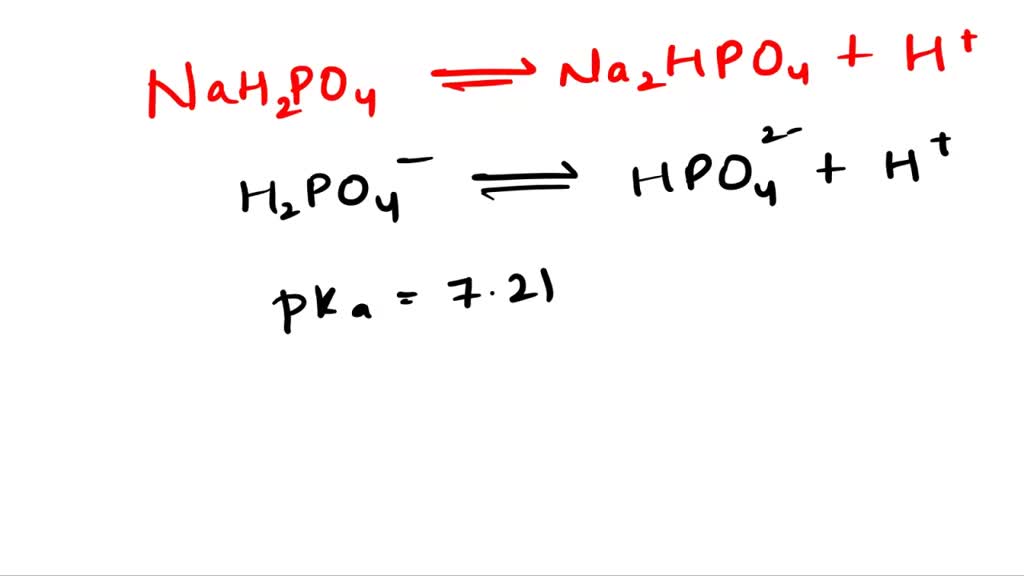
SOLVED: 1. Why is the equilibrium between the acid NaH2PO4, and its conjugate base Na2HPO4, a suitable buffer for maintaining intracellular pH (pH 6.9-7.3)?

OneClass: Equal molar quantities of sodium hydroxide and sodium hydrogenphosphate (Na2HPO4) are mixed...

Calculate the pH of a buffer solution obtained by dissolving 25.0 g of KH2PO4(s) and 38.0 g of Na2HPO4(s) in water and then diluting to 1.00 L. | Homework.Study.com
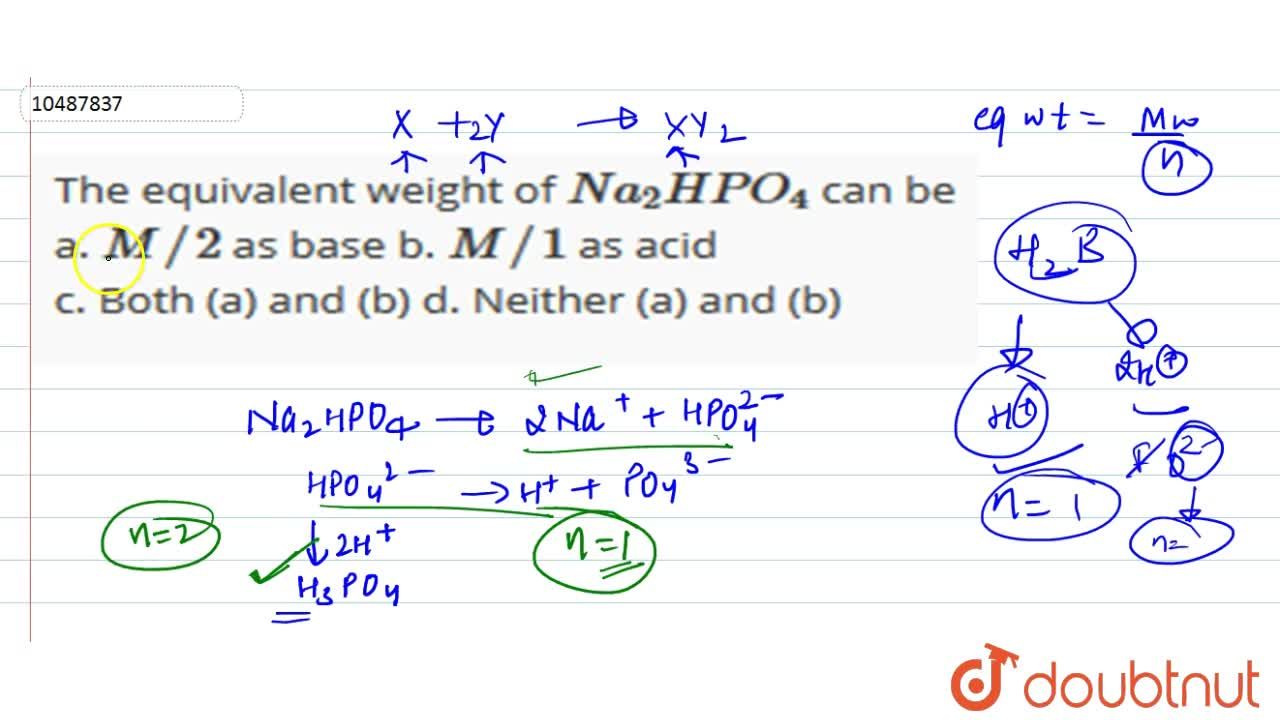
The equivalent weight of Na(2) HPO(4) can be a. M//2 as base b. M//1 as acid c. Both (a) and (b) d. Neither (a) and (b)